
The United Laboratories International Holdings Limited
(Incorporated in the Cayman Islands with limited liability)


[For Immediate Release]
Financial Highlights
(24 August 2022 - Hong Kong) The United Laboratories International Holdings Limited ("TUL", the "Company" or the "Group"; Stock code: 3933), one of the leading pharmaceutical product manufacturers in the PRC, announced today its unaudited interim results for the six months ended 30 June 2022 (the "Period").
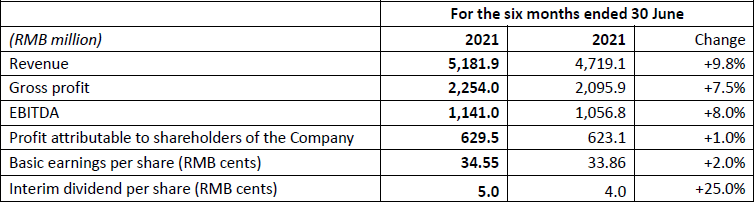
In the first half of 2022, the Group's revenue increased by 9.8% year on year to RMB5,181.9 million. Gross profit was RMB2,254.0 million, up 7.5% year on year. EBITDA was approximately RMB1,141.0 million, representing a year-on-year increase of 8.0%. Profit attributable to shareholders of the Company increased by 1.0% year on year to RMB629.5 million. Basic earnings per share amounted to RMB34.55 cents. The board of directors recommends an interim dividend of RMB5.0 cents per share.
During the Period, the Group's finished products recorded total revenue of RMB1,887.7 million. The sales revenue of the insulin series was RMB619.7 million. In particular, recombinant human insulin injection (USLIN) achieved sales revenue of RMB347.8 million, with sales volume increasing by 5.4% year on year. Glargine insulin injection (USLEN) achieved sales revenue of RMB236.7 million, with sales volume increasing by 40.5% year on year. Insulin aspart injection and insulin aspart 30 injection recorded sales revenue of RMB35.2 million. Six groups of our insulin products won the bids in the sixth batch of the national centralised procurement of drugs (for insulin only) (the "Special Centralised Procurement of Insulin"), covering the mealtime, basal and premixed groups of human insulin and insulin analogues. In May 2022, the results of the special centralised procurement of insulin were implemented in all provinces and cities across China. As a result, the decline in the price of insulin products and our corporate customers' tightened inventory management led to a year-on-year decrease in the profit margin of the Group's diabetes products. Ushering in a new chapter with the special centralised procurement of insulin, the Group will continue to expand its product sales across the country, increase market share and enhance brand influence, aiming to improve profits with increased scale, benefit the vast number of diabetic patients and accelerate the process of replacing imported drugs with domestic drugs in the field of diabetes.
As for other finished products, sales revenue of antibiotic products increased by 3.2% year on year to RMB1,166.5 million. In particular, piperacillin sodium and tazobactam sodium for injection recorded sales revenue of RMB315.0 million, and amoxicillin capsules recorded sales revenue of RMB210.0 million.
The Group is also stepping up the development of its veterinary drug business. During the Period, veterinary drugs recorded sales revenue of RMB206.6 million. In July 2022, The United Animal Healthcare (Inner Mongolia) Co., Ltd. (內 蒙 古 聯 邦 動 保 藥 品 有 限 公 司) ("United Animal Healthcare"), a wholly owned subsidiary of the Company, and Muyuan Foods Co., Ltd. (牧 原 食 品 股 份 有 限 公 司) ("Muyuan Foods") jointly established Henan Lianmu Veterinary Medicine Co., Ltd. (河 南 聯 牧 獸 藥 有 限 公 司). Based on years of cooperation between the two companies, the Company will fully utilize United Animal Healthcare's strengths in the R&D, production and technology, while leveraging on the brand influence, marketing network and resources of Muyuan Foods to expand its business. Moreover, United Animal Healthcare is committed to creating the most comprehensive veterinary drug series of β-lactam products and enzymatic preparation products in China. At present, it has more than 100 types of veterinary drugs that meet national standards, more than 30 types of feed additives, additive premixes and feed products. United Animal Healthcare also has 19 in-house R&D projects and eight co-development projects. The Group will continue to enhance its overall competitiveness in the field of veterinary drugs and strive to build a leading brand in the animal healthcare industry in China.
During the Period, the Group's intermediate products and bulk medicine recorded external sales revenue of RMB740.8 million and RMB2,553.4 million, representing a decrease of 9.9% and an increase of 29.7%, respectively. The significant increase in sales revenue of bulk medicine was mainly due to continued increase in sales price. The sales price of intermediate products such as 6-APA continued to climb, and the sales revenue of bulk medicine such as amoxicillin grew steadily. The Group's export sales recorded a revenue of RMB1,052.9 million, accounting for 20.3% of the Group's revenue. The Group continued to hold a leading position in the domestic and export market of intermediate products and bulk medicine.
During the period, the Group invested a total of RMB242.6 million in the R&D of pharmaceutical products, with a year-on-year increase of 19.4% in R&D expenses. At present, various R&D projects are progressing smoothly, with 32 new products under development, 12 of which are Class 1 new drugs. The Group has established a comprehensive R&D system, including multiple platforms such as biological drugs, chemical drugs, innovative drugs, clinical research centre and external cooperation, with a focus on the fields of endocrine, autoimmune, ophthalmology. Our projects include insulin analogue, GLP-1 receptor agonists such as semaglutide injection, gastrointestinal hormone analogue with multi-target effects, and JAK1 selective inhibitor for the treatment of rheumatoid arthritis. In particular, insulin degludec/insulin aspart injection received the notice of approval for clinical trial from the National Medical Products Administration in June 2022. Compared with the pre-mixed insulin analogues currently used in clinical practice, insulin degludec/insulin aspart injection can better simulate the physiological insulin secretion pattern and provide a safer and more effective glucose-lowering treatment, offering a more ideal choice for diabetic patients to achieve comprehensive blood glucose control. The Group is the second company in China to obtain clinical approval for this biosimilar drug, giving it another fist-mover advantage in the R&D of diabetes drugs.
The Group also achieved solid progress in the Quality and Efficacy Consistency Evaluation of Generic Drugs ("Consistency Evaluation"). During the Period, the Group's Biapenem for Injection (specification: 0.3g), Cefuroxime Tablets (specification: 0.125g) and Sodium Piperacillin Tazobactam for Injection (specification: 4.5g) passed the consistency evaluation successively. In the future, the Group will continue to press ahead with the R&D and consistency evaluation of new drugs to provide patients with safer and quality drug choices.
As part of its efforts to accelerate the R&D of biological drugs, the Group unveiled United Biotechnology (Hengqin) Co., Ltd. (聯 邦 生 物 科 技 (珠 海 橫 琴) 有 限 公 司) ("United Biotechnology"), a wholly owned subsidiary of the Group, in the Guangdong-Macao In-Depth Cooperation Zone in Hengqin, Guangdong Province in April 2022. As our biopharmaceutical R&D centre, United Biotechnology specialises in the R&D of energy metabolism, inflammation and autoimmune drugs, with a focus on the development of high-end biopharmaceuticals for the treatment of major chronic diseases, and will gradually develop itself into a professional organisation for chronic disease management. In the future, the Group will strengthen international cooperation and exchanges and introduce projects to actively promote the progress of new drug projects and further enhance its competitiveness in the biopharmaceutical industry.
In terms of finance, the Group reduced its finance costs by adjusting the borrowings mix of domestic and offshore borrowings, and continuously optimise its financial structure to improve its liquidity. During the Period, the Group's finance costs decreased to RMB21.8 million, representing a year-on-year decrease of 32.6%. As at 30 June 2022, the Group's net cash and bank balances amounted to RMB1,014.8 million and its financial position remained strong.
Looking ahead, Mr. Tsoi Hoi Shan, Chairman of the Group said, "In the future, the Group will continue to allocate more resources in the R&D of new drugs and the expansion of product lines to enhance our R&D capabilities and develop innovative products with market potential, thereby adding momentum to the Group's long-term sustainable development. Adhering to its mission of 'Making Life More Valuable,' the Group will seize the opportunities arising from the changes in China's pharmaceutical industry, continue to drive the development of its core businesses, and leverage on its R&D strengths to actively explore new growth momentum, thus creating more values for shareholders and society."
| Company Information Listed on the Stock Exchange of Hong Kong in June 2007, TUL is one of the leading comprehensive pharmaceutical companies in China, principally engaged in the R&D, manufacturing and selling of finished products, bulk medicines and intermediate products. The Group has six production bases, and its sales teams for intermediate products, bulk medicines and finished products formed a broad sales network covering China and the rest of the world. Currently, TUL is one of the few pharmaceutical companies in China that owns both second and third generation insulin products. The Group is presently a component of the Hang Seng Composite Index Series. |
For further enquiries, please contact:
iPR Ogilvy Ltd.
Tina Law / Joann Fang / Kelvin Tang
Tel: (852) 2136 6181 / 3920 7619
Fax: (852) 3170 6606
Email: tul@iprogilvy.com
| © Copyright 1996-2025 irasia.com Ltd. All rights reserved. |
|
DISCLAIMER: irasia.com Ltd makes no guarantee as to the accuracy or completeness of any
information provided on this website. Under no circumstances shall irasia.com Ltd be liable
for damages resulting from the use of the information provided on this website.
TRADEMARK & COPYRIGHT: All intellectual property rights subsisting in the contents of this website belong to irasia.com Ltd or have been lawfully licensed to irasia.com Ltd for use on this website. All rights under applicable laws are hereby reserved. Reproduction of this website in whole or in part without the express written permission of irasia.com Ltd is strictly prohibited. TERMS OF USE: Please read the Terms of Use governing the use of our website. |